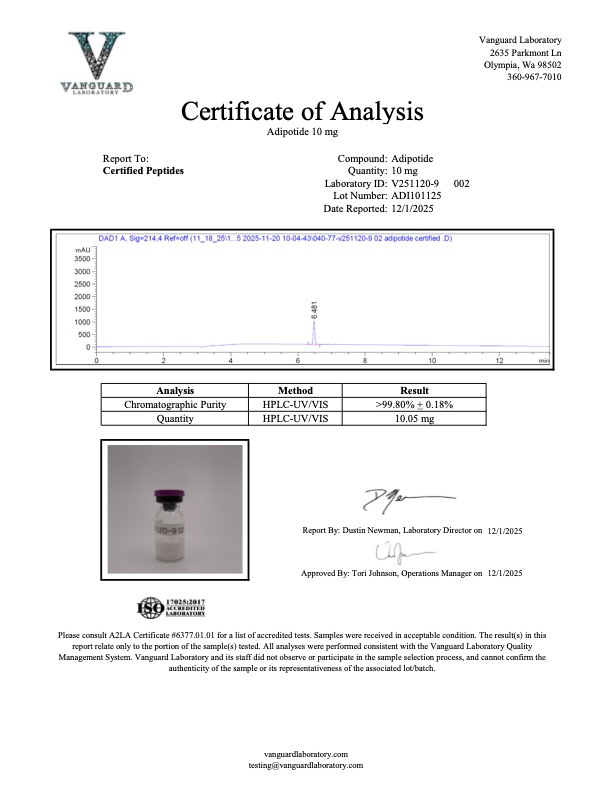
Adipotide is a vascular-targeting research peptide designed to selectively induce apoptosis within the microvasculature of white adipose tissue. By disrupting blood flow to adipocytes, the compound facilitates a programmed reduction of adipose mass through nutrient deprivation at the cellular level. Its mechanism of action is non-hormonal, distinguishing it from incretin-based agents such as GLP-1, GIP, or dual/triple agonists. Unlike those pathways, Adipotide does not rely on appetite modulation and therefore does not exhibit the characteristic gastrointestinal or centrally mediated side effects commonly associated with incretin mimetics.
Preclinical data indicate that Adipotide can promote a measurable reduction in both visceral and subcutaneous adipose tissue, with a comparatively stronger effect on visceral fat due to its higher vascular density and therefore greater susceptibility to targeted vascular apoptosis.
In research models, Adipotide has also been associated with several metabolic and cellular-level findings, including:
- Enhanced insulin sensitivity
- Reduced fasting glucose concentrations
- Decreases in circulating triglycerides
- Down-regulation of inflammatory cytokine activity
- Attenuation of low-grade systemic inflammation
- Improved leptin signaling dynamics
- Potential increases in mitochondrial efficiency and metabolic throughput
- Because of its vascular-directed mechanism, Adipotide is sometimes studied alongside AMPK activators, mitochondrial support agents, or metabolic modulators to evaluate possible synergistic effects on adipocyte turnover and energy utilization.